Chapter 01 Review Problems
Fundamentals
Classify each statement as being either quantitative or qualitative.
- This homework took a good amount of time to complete.
- This homework took 50 minutes to complete.
- This school has a beautiful campus.
Solution
Answer:
- qualitative
- quantitative
- qualitative
Concept: classifying data
The key difference between these two types of observations lies in what they describe.
- A qualitative observation describes a quality or characteristic. It is often subjective and based on the senses (color, texture, taste, or an opinion like “good” or “beautiful”). Statements (a) and (c) are qualitative because “a good amount” and “beautiful” are descriptive judgments, not numerical measurements.
- A quantitative observation describes a quantity or amount. It is objective and always involves a number with a unit of measurement. Statement (b) is quantitative because it provides a specific, measured value: 50 minutes.
Which statement best explains the difference between a theory and a law?
- A law is truth whereas a theory is mere speculation.
- A law summarizes a series of related observations, while a theory gives the underlying reasons for them.
- A theory describes what nature does; a law explains why nature does it.
Solution
Answer: B
Concept: scientific method
A scientific law summarizes what happens in a series of related observations, while a scientific theory provides the underlying explanation for why it happens.
A scientific law…
- summarizes a body of observations and can be used to make predictions.
- is a scientifically proven theory.
- provides a detailed explanation for why a phenomenon occurs.
- is a hypothesis that has not yet been disproven.
- is absolute and cannot be modified under any circumstances.
Solution
Answer: A
Concept: scientific method
A scientific law uses a concise statement or mathematical equation to summarize a large body of experimental observations and describe what happens in a natural phenomenon (e.g., the Ideal Gas Law). This allows it to predict future outcomes under similar conditions.
- and (C) are incorrect because they describe a scientific theory, which is a framework that explains why a law holds true.
- is incorrect because a law is a well-established principle, far beyond the initial stage of a hypothesis.
- is incorrect because all scientific knowledge, including laws, is provisional and subject to refinement if new, contradictory evidence is discovered.
All statements about scientific theories are true except
- they explain why nature behaves the way it does.
- they must have the ability to make predictions on future behavior.
- they should use observations to test the theory.
- they are derived from hypothesis.
- they are speculation.
Solution
Answer: E
Concept: scientific method
Option E is the correct answer because it reflects a common, everyday misuse of the word “theory.” In science, a theory is a robust, well-substantiated, and predictive framework, which is the opposite of speculation.
The other statements are all true characteristics of a scientific theory:- (a) A theory’s primary purpose is to explain the underlying reasons, or the “why,” behind a set of observations.
- (b) A powerful theory is not just explanatory; it must also have predictive power.
- (c, d) A theory is built upon one or more supported hypotheses and is continuously tested and refined against new observations.
Which of the following statements is the best example of a scientific hypothesis?
- The boiling point of water at sea level is 100 °C.
- A sealed jar of meat will eventually contain maggots because life arises from non-living matter.
- An iron bar rusts when left in a damp environment.
- The density of lead is greater than the density of aluminum.
- A sugar cube dissolves faster in hot water than in cold water.
Solution-
Answer: B
Concept: scientific method
A hypothesis is a tentative and testable explanation for a phenomenon. It proposes a “why” or “how” that can be investigated through experimentation, and it must be falsifiable.
- (B) is the best example of a hypothesis. It provides a testable explanation (“life arises from non-living matter”) for an observation (maggots appearing in meat). This was a real historical hypothesis that was famously tested and ultimately falsified by experiments like those of Francesco Redi and Louis Pasteur.
The other statements are all observations or established scientific facts, not tentative explanations. They describe what happens, but do not propose a mechanism for why it happens.
Which of the following substances is a heterogeneous mixture?
- rubbing alcohol
- sweetened tea
- concrete
- brass (an alloy of copper and zinc)
Solution
Answer: C
Concept: mixtures
A heterogeneous mixture has a non-uniform composition, with visibly distinct components or phases.
- (C) Concrete is the correct answer. You can see the individual components, such as sand, gravel, and cement, are not uniformly distributed.
The other options are all homogeneous mixtures. Rubbing alcohol and sweetened tea are solutions, and brass is a solid solution called an alloy. In all three, the components are mixed evenly at the atomic or molecular level.
Which of the following can be classified as a heterogeneous mixture?
- air
- copper wire
- salt water
- sand in water
- distilled water
Solution
Answer: D
Concept: classification of Matter
A heterogeneous mixture has a non-uniform composition with visibly distinct components.
- (D) Sand in water is the correct answer. The solid sand particles and the liquid water are separate phases that do not mix uniformly.
The other options are incorrect for the following reasons:
- (A) Air and (C) salt water are homogeneous mixtures (solutions), as their components are mixed evenly at the molecular level.
- (B) Copper wire and (E) distilled water are pure substances (an element and a compound, respectively), not mixtures.
Which of the following describes a process that requires a chemical change?
- Separating sand from gravel by using a sieve.
- Obtaining pure water from a salt water solution by evaporation and condensation.
- Separating a mixture of different colored inks on a piece of paper.
- Obtaining pure iron and pure sulfur from a sample of iron(II) sulfide.
- Separating differently sized proteins from a solution using a centrifuge.
Solution
Answer: D
Concept: physical vs. chemical changes
A chemical change (a chemical reaction) is required to break the chemical bonds that hold a compound together. A physical change is sufficient to separate the components of a mixture, as the components are not chemically bonded.
- (D) Iron(II) sulfide (FeS) is a compound. The iron and sulfur atoms are joined by strong chemical bonds. To separate them into pure iron and pure sulfur, these bonds must be broken through a chemical reaction.
The other options all describe the separation of mixtures using physical means, which do not alter the chemical identity of the components:
- (A) Sieving separates components based on a difference in particle size.
- (B) Evaporation and condensation (distillation) separates components based on a difference in boiling point.
- (C) Chromatography separates components based on differences in their attraction to a stationary phase.
- (E) Centrifugation separates components based on a difference in density or mass.
Which of the following combinations, when mixed thoroughly, will result in a homogeneous mixture? (Select all that apply)
- sand and water
- sugar and water
- oil and water
- table salt and water
- ethanol and water
Solution
Answer: B, D, E
Concept: homogeneous vs. heterogeneous mixtures
A homogeneous mixture (or solution) is formed when substances combine to create a mixture with a uniform composition down to the molecular level. A heterogeneous mixture has a non-uniform composition with visibly distinct components.
The following combinations form homogeneous mixtures:
- (B) Sugar and water: Sugar dissolves completely in water to form a uniform solution.
- (D) Table salt and water: Salt dissociates in water to form a uniform saline solution.
- (E) Ethanol and water: Ethanol is completely miscible with water, forming a uniform solution.
The other combinations form heterogeneous mixtures:
- (A) Sand and water: Sand does not dissolve and remains as a separate solid phase.
- (C) Oil and water: Oil is immiscible with water and will form a separate layer.
Which property is characteristic of a gas but not of a liquid or a solid?
- Its particles are in constant, random motion.
- It takes on the shape of its container.
- It is highly compressible.
- It is composed of molecules rather than atoms.
- Its density is dependent on temperature.
Solution
Answer: C
Concept: states of matter
The defining difference between gases and condensed phases (liquids and solids) is the large amount of empty space between gas particles. This space is what allows a gas to be easily compressed.
- (C) It is highly compressible. This is the correct answer. The volume of a gas can be significantly decreased by applying pressure because its particles are very far apart. Solids and liquids are considered incompressible because their particles are already in close contact.
The other statements are incorrect for the following reasons:
- (A) The particles in a liquid are also in constant, random motion.
- (B) Liquids also take on the shape of the portion of the container they occupy.
- (D) Solids, liquids, and gases can all be composed of molecules (e.g., solid ice, liquid water, gaseous steam are all H2O molecules).
- (E) The densities of solids and liquids are also dependent on temperature (most substances expand when heated), although the effect is much less dramatic than in gases.
Identify the incorrect statement.
- Helium in a balloon is an element
- Paint is a mixture
- Tap water is a compound
- Mercury in a thermometer is an element
Solution
Answer: C
Concept: classification of matter
The statement that tap water is a compound is incorrect.
- A compound is a pure substance consisting of two or more elements chemically bonded in a fixed ratio (e.g., pure water, H2O).
- A mixture is a combination of two or more substances that are not chemically bonded.
Tap water is a homogeneous mixture (a solution). While it is mostly the compound water (H2O), it also contains many other dissolved substances, such as minerals (ions like Ca2+, Mg2+) and gases (O2, CO2). Because its composition is not a fixed ratio of H and O atoms alone, it is a mixture, not a compound.
The other statements are correct:
- (A) Helium (He) and (D) mercury (Hg) are both pure elements.
- (B) Paint is a complex mixture of various substances like pigments, binders, and solvents.
A student observes five processes. Which of the choices includes all the processes that are chemical changes?
- Melting a block of solid ice.
- Cooking an egg.
- Dissolving sugar in hot tea.
- A piece of magnesium ribbon burning with a bright white light.
- An antacid tablet fizzing after being dropped in water.
- II, IV, and V
- IV and V
- II only
- I, II, III, IV, and V
- I and III
Solution
Answer: A
Concept: physical vs. chemical changes
A chemical change results in the formation of new chemical substances with different properties. A physical change alters the form or appearance of a substance but not its chemical identity.
The processes that are chemical changes are:
- (II) Cooking an egg: The heat denatures the proteins in the egg, fundamentally changing their chemical structure. This change is irreversible.
- (IV) Burning magnesium: This is a combustion reaction where magnesium (Mg) reacts with oxygen (O2) to form a new compound, magnesium oxide (MgO).
- (V) An antacid tablet fizzing: This is a chemical reaction, typically between citric acid and sodium bicarbonate, that produces carbon dioxide gas (the fizzing).
The processes that are physical changes are:
- (I) Melting ice: This is a phase change from solid water to liquid water. The substance is still H2O.
- (III) Dissolving sugar: The sugar molecules disperse into the tea, but they remain individual sugar molecules. The sugar can be recovered by evaporating the water.
Which of the following observations is a result of a chemical change, not a physical change?
- The sublimation of dry ice (solid CO2) into a gas.
- A silver spoon tarnishing over time.
- The distillation of ethanol from a fermented mash.
- I only
- II only
- III only
- II and III
- I and III
Solution
Answer: B
Concept: physical vs. chemical changes
A chemical change results in the formation of new chemical substances. A physical change alters a substance’s form but not its chemical identity.
- (II) A silver spoon tarnishing is a chemical change. The silver (Ag) reacts with sulfur compounds in the air to form a new compound, silver sulfide (Ag2S), which is the black tarnish.
The other processes are physical changes:
- (I) Sublimation is a phase change from a solid directly to a gas. The substance is still CO2.
- (III) Distillation is a physical separation technique that separates substances based on differences in their boiling points.
A chemist is studying the properties of iron. Which of the following are physical properties of iron?
- It is a solid at 25 °C.
- It can be drawn into a wire (it is ductile).
- It rusts when exposed to air and water.
- It has a melting point of 1538 °C.
- It can be magnetized.
- I, II, IV, and V
- III only
- I and IV only
- I, II, III, IV, and V
- II, III, and V
Solution
Answer: A
Concept: physical vs. chemical properties
A physical property is a characteristic of a substance that can be observed or measured without changing its chemical composition (e.g., color, density, melting point, ductility). A chemical property describes the ability of a substance to undergo a specific chemical change (e.g., its reactivity with other substances).
The physical properties listed are:- (I) State of matter at room temperature is a physical property.
- (II) Ductility (the ability to be drawn into a wire) is a physical property.
- (IV) Melting point is a classic physical property.
- (V) Magnetism is a physical property.
- (III) Rusting describes a chemical reaction where iron combines with oxygen to form a new substance, iron oxide. This is a chemical property.
Which of the following statements describes a chemical property of water?
- It is a colorless, odorless liquid at room temperature.
- It freezes at 0 °C and boils at 100 °C at standard pressure.
- It can be decomposed into hydrogen and oxygen gas by passing an electric current through it.
- Its density is approximately 1.00 g cm−3 at 4 °C.
- It can dissolve many ionic and polar substances, such as salt and sugar.
Solution
Answer: C
Concept: physical vs. chemical properties
A chemical property describes the ability of a substance to undergo a specific chemical change. A physical property is a characteristic that can be observed without changing the substance’s chemical identity.
- (C) The decomposition of water into new substances (hydrogen and oxygen) is a chemical reaction (electrolysis). The ability to undergo this reaction is a chemical property of water.
The other statements all describe physical properties of water:
- (A) Color and odor are physical properties.
- (B) Freezing and boiling points are physical properties.
- (D) Density is a physical property.
- (E) Solubility (its ability to act as a solvent) is a physical property because dissolving a substance like sugar does not change the chemical nature of the water or the sugar.
Which of the following processes is a chemical change?
- A candle wick burning.
- Chopping a log into smaller pieces.
- Water turning into steam.
- Dissolving sugar in water.
- Bending a copper wire.
Solution
Answer: A
Concept: physical vs. chemical changes
A chemical change results in the formation of new chemical substances, often accompanied by indicators like the production of heat, light, or a gas. A physical change alters a substance’s appearance or state, but not its chemical identity.
- (A) A candle wick burning is a chemical change. The wick and wax undergo combustion, reacting with oxygen to form new substances like carbon dioxide and water vapor.
The other options are all physical changes:
- (B) Chopping a log and (E) bending a wire are changes in shape and form.
- (C) Water turning into steam is a phase change (boiling).
- (D) Dissolving sugar is a physical process of mixing.
Which of the following substances would you expect to be the most dense under standard conditions?
- A block of solid aluminum.
- A sample of liquid mercury.
- A tank of compressed nitrogen gas.
- A sample of liquid ethanol.
Solution
Answer: B
Concept: density and states of matter
Density is a measure of mass per unit volume. For most substances, the solid phase is denser than the liquid phase, and the liquid phase is significantly denser than the gaseous phase. Furthermore, metals are generally very dense materials.
- (B) Liquid mercury is the correct answer. It is a metal, and with a density of approximately 13.6 g cm–3, it is one of the densest common substances. It is significantly denser than most other liquids and solids.
Comparing the other options:
- (A) Solid aluminum is a relatively dense metal (~2.7 g cm−3), but it is much less dense than mercury.
- (C) Nitrogen gas, like all gases, has a very low density because its particles are far apart.
- (D) Liquid ethanol has a density lower than that of water (~0.79 g cm−3).
Which of the following common substances has the lowest density at room temperature?
- Iron
- Water
- Air
- Wood (Oak)
Solution
Answer: C
Concept: density and states of matter
Density is a measure of mass per unit volume. In general, gases are far less dense than liquids and solids because the particles in a gas are spread very far apart.
- (C) Air is the correct answer. As a gas, it has a very low density (approximately 0.0012 g cm−3 at sea level).
The other options are all condensed phases (liquids or solids) and are therefore much denser:
- (A) Iron is a dense solid metal (~7.87 g cm−3).
- (B) Water is a liquid with a density of approximately 1.00 g cm−3.
- (D) Oak wood is a solid with a density less than water (~0.75 g cm−3), but it is still hundreds of times denser than air.
Math Basics and Significant Figures
Evaluate this expression and report the answer to the correct number of significant figures.
\[ (104.2 - 98.4) \times 3.518 \]
Solution
Answer: 16
Concept: significant figures and multi-step calculations
According to the order of operations, we must solve the expression in the parentheses first.
\[ \begin{align*} (104.\bar{2} - 98.\bar{4}) \times 3.51\bar{8} &= (5.\bar{8}) \times (3.51\bar{8}) \\[1.5ex] &= 20.4\bar{0}44 \\[1.5ex] &= 16 \end{align*} \]
Write the following numbers in normalized scientific notation to three significant figures.
- 0.000 325 34
- 3 438.001
- 0.034 801 0
- 13.732 4
- 0.000 050 555
Solution
Answer:
- 3.25 × 10–4
- 3.44 × 103
- 3.48 × 10–2
- 1.37 × 101
- 5.06 × 10–5
Concept: scientific notation and rounding
How many significant figures are in each number?
- 433 km
- 4 pennies
- 2.12 × 105 m
- 0.000 88 s
- 2.560 0 km
- 32 000 m
Solution
Answer:
- 3
- ∞ (obtained via counting)
- 3
- 2
- 5
- ambiguous but considered to be 2 for this class
Concept: significant figures
Determine the number of significant figures for each of the following numbers.
- 3 200
- 0.320 0
- 3 200 pencils
- 00.320 0
- 3 200.0 g
- 3 200.010 0
Solution
Answer:
- ambiguous (but considered to for most classes)
- 4
- ∞ (obtained via counting)
- 4
- 5
- 8
Concept: significant figures
How many significant figures are in the answer to the following calculation?
\[\left ( 29.0025 + 0.2 \right ) \left (6.134 - 6.101 \right ) / 5.196\times 10^{-2}\]
Solution
Answer: 2
Concept: significant figures
\[ \begin{align*} (29.002\bar{5} + 0.\bar{2})(6.13\bar{4}-6.10\bar{1}) / 5.19\bar{6}\times 10^{-2} &= (29.\bar{2}02)(0.03\bar{3}00) / 5.19\bar{6}\times 10^{-2} \\[1.5ex] &= (0.9\bar{6}366) / 5.19\bar{6}\times 10^{-2} \\[1.5ex] &= 1\bar{8}.54 \\[1.5ex] &= 18 \end{align*} \]
Note: The answer is 18 when using the round-half-to-even rule. If using the round-up rule, the answer would be 19. In either case, both have 2 significant figures.
What answer should be reported, with the correct number of significant figures, for the following calculation? Report your answer in normalized scientific notation.
\[\left ( 433.621 - 333.9 \right ) \times 11.900\]
Solution
Answer: 1.19 × 103
Concept: significant figures
\[ \begin{align*} \left ( 433.62\bar{1} - 333.\bar{9} \right ) \times 11.90\bar{0} &= \left ( 99.\bar{7}21 \right ) \times 11.900 \\[1.5ex] &= 11\bar{8}6.6 \\[1.5ex] &= 1~1\bar{9}0 \\[1.5ex] &= 1.19\times 10^3 \end{align*} \]
Measurements and Conversions
What appropriate volume (in mL) of liquid does this measurement on a graduated cylinder convey?
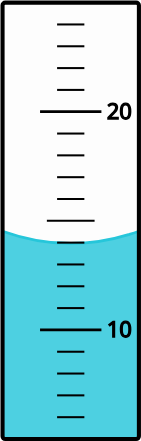
Solution
Answer: 14.0 mL; the zero in the tenths-place is the uncertain (estimated) number
Concept: uncertainty; measurement
A student performs an experiment to determine the density of a sugar solution. They obtain the following results: 12.11 g mL–1, 12.81 g mL–1, 12.95 g mL–1, 12.75 g mL–1. The actual value for the density of the sugar solution is 12.75 g mL–1. Determine if the following statements are true or false.
- The results are precise.
- The results are accurate.
Solution
Answer: A and B are both false.
Concept: accuracy and precision
A student performs an experiment to determine the density of a metal. The accepted, true density of the metal is 8.96 g cm−3. The student’s results from four trials are:
6.15 g cm−3, 6.16 g cm−3, 6.15 g cm−3, 6.17 g cm−3
Which of the following statements best describes these results?
- The results are accurate and precise.
- The results are accurate but not precise.
- The results are precise but not accurate.
- The results are neither accurate nor precise.
Solution
Answer: C
Concept: accuracy and precision
- Accuracy refers to how close a set of measurements is to the true or accepted value.
- Precision refers to how close a set of measurements are to each other (reproducibility).
Let’s analyze the student’s results:
Precision: The results are all very close to each other, clustered in a narrow range. This indicates high precision.
Accuracy: The results are all clustered around 6.16 g cm−3, which is very far from the true value of 8.96 g cm−3. This indicates low accuracy.
Conclusion: The results are precise but not accurate. This pattern often suggests a systematic error in the experiment, such as a miscalibrated instrument.
Perform the following conversions below. Report your answers in normalized scientific notation to the appropriate number of significant figures.
- 145.21 kg to mg
- 0.490 nm to m
- 42.1 cm3 to L
- 1.73 × 105 m3 to cm3
Solution
Answer:
- 1.4521 × 108 mg
- 4.90 × 10–10 m
- 4.21 × 10–2 L
- 1.73 × 1011 cm3
Concept: significant figures; scientific notation
\[ \begin{align*} \mathbf{A.}~ &145.2\bar{1}~\mathrm{kg} \left ( \dfrac{10^3~\mathrm{g}}{\mathrm{kg}} \right ) \left ( \dfrac{10^3~\mathrm{mg}}{\mathrm{g}} \right ) = 1.4521\times 10^{8}~\mathrm{mg} \\[1.5ex] \mathbf{B.}~ &0.49\bar{0}~\mathrm{nm} \left ( \dfrac{\mathrm{m}}{10^9~\mathrm{nm}} \right ) = 4.90\times 10^{-10}~\mathrm{m} \\[1.5ex] \mathbf{C.}~ &42.\bar{1}~\mathrm{cm}^3 \left ( \dfrac{\mathrm{mL}}{\mathrm{cm}^3} \right ) \left ( \dfrac{\mathrm{L}}{10^3~\mathrm{mL}} \right ) = 4.21\times 10^{-2}~\mathrm{L}\\[1.5ex] \mathbf{D.}~ &1.7\bar{3}\times 10^5~\mathrm{m}^3 \left ( \dfrac{\mathrm{10^2~cm}}{\mathrm{m}} \right )^3 = 1.73\times 10^{11}~\mathrm{cm^3} \end{align*} \]
What is the length (in mm) of 4.20 cm?
Solution
Answer: 42.0 mm
Concept: metric conversions
\[\begin{align*} 4.2\bar{0}~\mathrm{cm} \left ( \dfrac{\mathrm{m}}{10^2~\mathrm{cm}} \right ) \left ( \dfrac{10^3~\mathrm{mm}}{\mathrm{m}} \right ) = 42.\bar{0}~\mathrm{mm} \end{align*}\]
Nitric acid is a very important industrial chemical and 46.3 billion pounds were produced in 2000. If the density of nitric acid is 11.85 lb gal–1, what is the volume (in L) in normalized scientific notation? 1 gal = 3.7854 L (inexact)
Solution
Answer: 1.48 × 1010 L
Concept: Imperial and metric conversions
\[\begin{align*} 4.6\bar{3}\times 10^{10}~\mathrm{lb} ~ \left ( \dfrac{1~\mathrm{gal}}{11.8\bar{5}~\mathrm{lb}} \right ) \left ( \dfrac{3.785\bar{4}~\mathrm{L}}{1~\mathrm{gal}} \right ) &= 1.4\bar{7}90\times 10^{10}~\mathrm{L} \\[1.5ex] &= 1.48\times 10^{10}~\mathrm{L} \end{align*}\]
Density
If the walls in a room are 955 ft2 in area, and a gallon of paint covers 15 yd2, how many gallons of paint are needed for the room? (3 ft = 1 yd)
Solution
Answer: 7.1 gal
Concept: conversions
\[\begin{align*} 95\bar{5}~\mathrm{ft^2} ~ \left ( \dfrac{\mathrm{yd}}{3~\mathrm{ft}} \right )^2 \left ( \dfrac{\mathrm{gal}}{1\bar{5}~\mathrm{yd^2}} \right ) &= 7.\bar{0}74~\mathrm{gal} \\[1.5ex] &= 7.1~\mathrm{gal} \end{align*}\]
A piece of metal ore has a mass of 9.25 g. When a student places it into a graduated cylinder containing water, the liquid level rises from 21.25 mL to 26.47 mL. What is the density (in g mL–1) of the ore?
Solution
Answer: 1.77 g mL–1
Concept: density; volume displacement
\[\begin{align*} V(\mathrm{ore}) &= V_{\mathrm{f}} - V_{\mathrm{i}} \\[1.5ex] &= 26.4\bar{7}~\mathrm{mL} - 21.2\bar{5}~\mathrm{mL} \\[1.5ex] &= 5.2\bar{2}~\mathrm{mL}\\[3ex] d(\mathrm{ore}) &= \dfrac{m}{V} \\[1.5ex] &= \dfrac{9.2\bar{5}~\mathrm{g}}{5.2\bar{2}~\mathrm{mL}} \\[1.5ex] &= 1.7\bar{7}20~\mathrm{g~mL^{-1}}\\[1.5ex] &= 1.77~\mathrm{g~mL^{-1}} \end{align*}\]
A sample containing 33.42 g of metal pellets is poured into a graduated cylinder that initially contains 12.7 mL of water. The final water level in the cylinder is 21.6 mL. What is the density (in g mL–1) of the metal?
Solution
Answer: 3.8 g mL–1
Concept: density; volume displacement
\[\begin{align*} \Delta V &= V_{\mathrm{final}} - V_{\mathrm{initial}} \\[1.5ex] &= 21.\bar{6}~\mathrm{mL} - 12.\bar{7}~\mathrm{mL} \\[1.5ex] &= 8.\bar{9}~\mathrm{mL}\\[3ex] d &= \dfrac{m}{V} \\[1.5ex] &= \dfrac{33.4\bar{2}~\mathrm{g}}{8.\bar{9}~\mathrm{mL}} \\[1.5ex] &= 3.\bar{7}55~\mathrm{g~mL^{-1}} \\[1.5ex] &= 3.8~\mathrm{g~mL^{-1}} \end{align*}\]
A rectangular block has the following dimensions: 2.5 cm × 3.9 cm × 10.1 cm. The mass of the block is 516.0 g. What is the volume (in cm3) and density (in kg m–3) of the block?
Solution
Answer: 9.8 cm3; 5.2 × 103 kg m–3
Concept: volume; density
\[\begin{align*} V &= l \times w \times h \\[1.5ex] &= 2.\bar{5}~\mathrm{cm} \times 3.\bar{9}~\mathrm{cm} \times 10.\bar{1}~\mathrm{cm} \\[1.5ex] &= 9\bar{8}.47~\mathrm{cm^3} \\[1.5ex] &= 9\bar{8}~\mathrm{cm^3} \\[3ex] d &= \dfrac{m}{V} \\[1.5ex] &= \dfrac{516.\bar{0}~\mathrm{g}}{9\bar{8}.47~\mathrm{cm^3}} \left ( \dfrac{\mathrm{kg}}{10^3~\mathrm{g}} \right ) \left ( \dfrac{10^2~\mathrm{cm}}{\mathrm{m}} \right )^3 \\[1.5ex] &= 5\bar{2}40~\mathrm{kg~m^{-3}} \\[1.5ex] &= 5.2\times 10^{3}~\mathrm{kg~m^{-3}} \end{align*}\]
At room temperature, elemental bromine (Br2) is a liquid with a density of 3.12 g cm–3. What is the mass (in g) of 115 mL of bromine and what volume (in mL) does 75.0 g of bromine occupy?
Solution
Answer: 359. g; 24.0 mL
Concept: volume; density
\[\begin{align*} d &= \dfrac{m}{V} \longrightarrow \\[1.5ex] m &= Vd \\ &= 11\bar{5}~\mathrm{mL} \left ( \dfrac{3.1\bar{2}~\mathrm{g}}{\mathrm{cm}^3} \right ) \left ( \dfrac{\mathrm{cm}^3}{\mathrm{mL}} \right ) \\[1.5ex] &= 35\bar{8}.8~\mathrm{g}\\[1.5ex] &= 359~\mathrm{g}\\[3ex] d &= \dfrac{m}{V} \longrightarrow \\[1.5ex] V &= \dfrac{m}{d} \\[1.5ex] &= \dfrac{75.0~\mathrm{g}}{3.12~\mathrm{g~cm^{-3}}} \left ( \dfrac{\mathrm{mL}}{\mathrm{cm}^3} \right ) \\[1.5ex] &= 24.\bar{0}38~\mathrm{mL} \\[1.5ex] &= 24.0~\mathrm{mL} \end{align*}\]
The Hindenburg contained 7.062 × 106 ft3 of hydrogen gas (d(H2) = 8.988 × 10–5 g mL–1 ). How much heavier (in kg) would the airship be if filled with air (d(air) = 1.225 × 10–3 g mL–1) instead? Report your answer in normalized scientific notation. (1 ft = 0.3048 m)
Solution
Answer: 2.270 × 105 kg
Concept: volume; density
\[\begin{align*} V &= 7.06\bar{2}\times 10^{6}~\mathrm{ft}^3 \left ( \dfrac{0.304\bar{8}~\mathrm{m}}{\mathrm{ft}} \right )^3 \left ( \dfrac{10^2~\mathrm{cm}}{\mathrm{m}} \right )^3 \left ( \dfrac{\mathrm{mL}}{\mathrm{cm^3}} \right ) \\[1.5ex] &= 1.99\bar{9}73\times 10^{11}~\mathrm{mL}\\[3.0ex] d &= \dfrac{m}{V} \longrightarrow \\[1.5ex] m(\mathrm{H_2}) &= V\,d(\mathrm{H_2}) \\[1.5ex] &= 1.99\bar{9}73\times 10^{11}~\mathrm{mL}~ \left ( \dfrac{8.98\bar{8}\times 10^{-5}~\mathrm{g}}{\mathrm{mL}} \right ) \left ( \dfrac{\mathrm{kg}}{10^3~\mathrm{g}} \right ) \\[1.5ex] &= 1.79\bar{7}35\times 10^{4}~\mathrm{kg}\\[3ex] m(\mathrm{air}) &= V\,d(\mathrm{air}) \\[1.5ex] &= 1.99\bar{9}73\times 10^{11}~\mathrm{mL}~ \left ( \dfrac{1.22\bar{5}\times 10^{-3}~\mathrm{g}}{\mathrm{mL}} \right ) \left ( \dfrac{\mathrm{kg}}{10^3~\mathrm{g}} \right ) \\[1.5ex] &= 2.44\bar{9}66\times 10^{5}~\mathrm{kg}\\[3ex] \Delta m &= m(\mathrm{air}) - m(\mathrm{H_2}) \\[1.5ex] &= 2.44\bar{9}66\times 10^{5}~\mathrm{kg} - 1.79\bar{7}35\times 10^{4}~\mathrm{kg} \\[1.5ex] &= 2.44\bar{9}66\times 10^{5}~\mathrm{kg} - 0.179\bar{7}35\times 10^{5}~\mathrm{kg} \\[1.5ex] &= (2.44\bar{9}66 - 0.179\bar{7}35) \times 10^5~\mathrm{kg} \\[1.5ex] &= 2.26\bar{9}92\times 10^5~\mathrm{kg} \\[1.5ex] &= 2.26\bar{9}92\times 10^{5}~\mathrm{kg} \\[1.5ex] &= 2.270\times 10^{5}~\mathrm{kg} \end{align*}\]
Temperature
Which of the following options is at the highest temperature?
- 71.4 °F
- 22.1 °C
- 294.65 K
Solution
Answer: B
Concept: temperature
This solution converts all temperatures to °C.
\[ \begin{align*} \textbf{A.}~~~ t\left ( ^{\circ}\mathrm{C} \right ) &= \dfrac{ [ t(^{\circ}\mathrm{F}) - 32 ]}{1.8} \\[1.5ex] &= \dfrac{ \left ( 71.\bar{4}~^{\circ}\mathrm{F} - 32\right ) }{1.8} \\[1.5ex] &= 21.\bar{8}88~^{\circ}\mathrm{C} \\[1.5ex] &= 21.9~^{\circ}\mathrm{C}\\[3ex] \textbf{B.}~~~ t\left ( ^{\circ}\mathrm{C} \right ) &= 22.\bar{1}~^{\circ}\mathrm{C} \\[1.5ex] &= 22.1~^{\circ}\mathrm{C}\\[3ex] \textbf{C.}~~~ t\left (^{\circ}\mathrm{C} \right ) &= T(\mathrm{K}) - 273.15 \\[1.5ex] &= 294.6\bar{5}~\mathrm{K} - 273.15 \\[1.5ex] &= 21.5\bar{0}~^{\circ}\mathrm{C} \\[1.5ex] &= 21.50 \end{align*} \]
Option B (22.1 °C) is the highest temperature.
The average daytime temperatures on Earth and Jupiter are 72 °F and 313 K, respectively. Which planet is hotter, on average, and by how much (in °C)?
Solution
Answer: Jupiter, 18 °C
Concept: temperature
\[\begin{align*} t_{\mathrm{Earth}}\left ( ^{\circ}\mathrm{C} \right ) &= \dfrac{ [ t_{\mathrm{Earth}}(^{\circ}\mathrm{F}) - 32 ]}{1.8} \\[1.5ex] &= \dfrac{\left ( 7\bar{2}~^{\circ}\mathrm{F} - 32 \right )}{1.8} \\[1.5ex] &= 2\bar{2}.22~^{\circ}\mathrm{C}\\[3ex] t_{\mathrm{Jupiter}}\left (^{\circ}\mathrm{C} \right ) &= T_{\mathrm{Jupiter}}(\mathrm{K}) - 273.15 \\[1.5ex] &= 31\bar{3}~\mathrm{K} - 273.15 \\[1.5ex] &= 3\bar{9}.85~^{\circ}\mathrm{C} \\[3ex] \Delta T &= t_{\mathrm{Jupiter}}\left (^{\circ}\mathrm{C} \right ) - t_{\mathrm{Earth}}\left (^{\circ}\mathrm{C} \right ) \\[1.5ex] &= 3\bar{9}.85~^{\circ}\mathrm{C} - 2\bar{2}.22~^{\circ}\mathrm{C}\\[1.5ex] &= 1\bar{7}.63~^{\circ}\mathrm{C} \\[1.5ex] &= 18~^{\circ}\mathrm{C} \end{align*}\]