Acids and Bases
Definitions

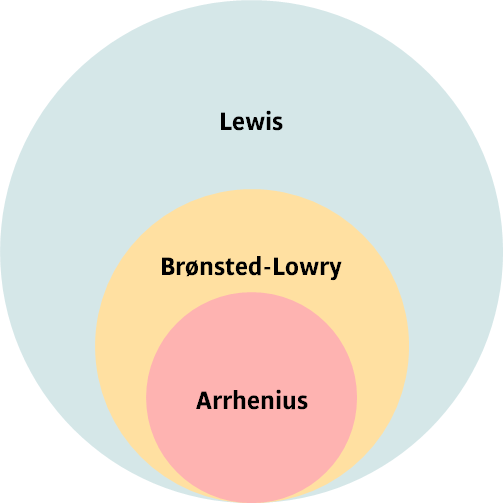
Arrhenius acid (green) – a substance that, when added to water, causes an increase in the molar concentration of hydronium ions ([H₃O⁺]) and a decrease in the molar concentration of hydroxide ions ([OH⁻]).
\[\begin{aligned} {\color{green}\mathrm{HA}}\mathrm{(aq)} + \mathrm{H_2O(l)} &\rightleftharpoons \mathrm{H_3O^+(aq)} + {\color{red}\mathrm{A^-}}\mathrm{(aq)} \end{aligned}\]
Arrhenius base (red) – a substance that, when added to water, causes an increase in the molar concentration of hydroxide ions ([OH⁻]) and a decrease in the molar concentration of hydronium ions ([H₃O⁺]).
\[\begin{aligned} \textrm{(by dissociation)} \quad \mathrm{NaOH}\mathrm{(s)} &\rightarrow \mathrm{Na^+(aq)} + {\color{red}\mathrm{OH^-}}\mathrm{(aq)} \\ \textrm{(by reaction)} \quad {\color{red}\mathrm{B}}\mathrm{(aq)} + \mathrm{H_2O(l)} &\rightleftharpoons \mathrm{OH^-(aq)} + {\color{green}\mathrm{HB^+}}\mathrm{(aq)} \end{aligned}\]
Brønsted-Lowry acid (green) – a molecular entity capable of donating a proton (hydron) to a base (a proton donor).
Brønsted-Lowry base (red) – a molecular entity capable of accepting a proton (hydron) from an acid (a proton acceptor).
A B-L reaction is a proton transfer. Water can act as either an acid or a base (water is amphoteric).
\[\begin{array}{ccccccc} {\color{green}\mathrm{HA}} & \!\!\!+\!\!\! & {\color{red}\mathrm{H_2O}} & \!\!\!\rightleftharpoons\!\!\! & {\color{green}\mathrm{H_3O^+}} & \!\!\!+\!\!\! & {\color{red}\mathrm{A^-}} \\ \small\text{B-L acid} & & \small\text{B-L base} & & \small\text{conj. acid} & & \small\text{conj. base} \end{array}\] \[\begin{array}{ccccccc} {\color{green}\mathrm{H_2O}} & \!\!\!+\!\!\! & {\color{red}\mathrm{B}} & \!\!\!\rightleftharpoons\!\!\! & {\color{green}\mathrm{HB^+}} & \!\!\!+\!\!\! & {\color{red}\mathrm{OH^-}} \\ \small\text{B-L acid} & & \small\text{B-L base} & & \small\text{conj. acid} & & \small\text{conj. base} \end{array}\]
The B-L theory also applies to non-aqueous systems:
\[\begin{array}{ccccc} {\color{green}\mathrm{HCl}}(\mathrm{g}) & \!\!\!+\!\!\! & {\color{red}\mathrm{NH_3}}(\mathrm{g}) & \!\!\!\rightleftharpoons\!\!\! & {\color{green}\mathrm{NH_4Cl}}(\mathrm{s}) \\ \small\text{B-L acid} & & \small\text{B-L base} & & \small\text{salt} \end{array}\]
Lewis acid (green) – a molecular entity that is an electron-pair acceptor.
Lewis base (red) – a molecular entity that is an electron-pair donor.
The Lewis theory is the most general acid-base theory because it defines reactions in terms of electron-pair transfer, allowing it to encompass reactions that do not involve protons.
\[\begin{array}{ccccc} {\color{green}\mathrm{BF_3}} & \!\!\!+\!\!\! & {\color{red}\mathrm{:NH_3}} & \!\!\!\rightarrow\!\!\! & \mathrm{F_3B{-}NH_3} \\ \small\text{Lewis acid} & & \small\text{Lewis base} & & \small\text{adduct} \end{array}\]
Here, the lone pair on nitrogen is donated to the electron-deficient boron atom.
Conjugate acid-base pairs
A deprotonated (−H+) acid results in a conjugate base. A protonated (+H+) base results in a conjugate acid.
\[{\color{green}\mathrm{HA}}\underset{+\mathrm{H}^+}{\overset{-\mathrm{H}^+}{\rightleftharpoons}} {\color{red}\mathrm{A^-}}\]
A protonated (+H+) base results in a conjugate acid. A deprotonated (−H+) acid results in a conjugate base.
\[{\color{red}\mathrm{B}} \underset{-\mathrm{H}^+}{\overset{+\mathrm{H}^+}{\rightleftharpoons}} {\color{green}\mathrm{HB^+}}\]
Classifications
Dissociation Constants
Notes
The acid dissociation constant, Ka, is the equilibrium constant for the ionization of an acid in water:
\[\mathrm{HA(aq)} + \mathrm{H_2O(l)}\rightleftharpoons \mathrm{H_3O^+(aq)} + \mathrm{A^-(aq)}\] \[K_{\mathrm{a}} = \dfrac{[\mathrm{H_3O^+}][\mathrm{A^-}]}{[\mathrm{HA}]}\]
This value is commonly expressed in logarithmic form as the pKa:
\[\mathrm{p}K_{\mathrm{a}} = -\log K_{\mathrm{a}}\]
Parent compound Type:
- A: acid
- B: base
- Amp: amphiprotic
Entries listed as bases contain pKa and Ka values associated with their conjugate acid. For example, ammonia (NH3) is a base and the listed values correspond to the ammonium ion (NH4+), the conjugate acid as determined by
\[\mathrm{NH_3(aq)} + \mathrm{H_2O(l)} \rightleftharpoons \mathrm{OH^-(aq)} + \mathrm{NH_4^+(aq)}\]