Acids and Bases
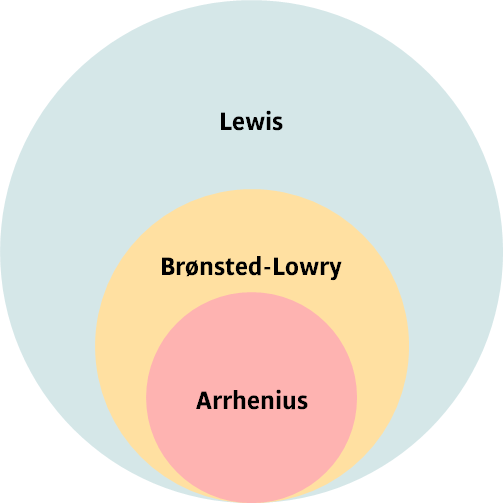
Acids and bases are special types of molecular compounds that are fundamental to chemistry, governing everything from the pH of our blood to the reactions in a car battery. Over the years, our understanding of what makes a substance an “acid” or a “base” has evolved. We will explore three key definitions, each one more general than the last, that together provide a complete picture of acid-base chemistry. The relationship between these theories is shown in the Venn diagram below.

Throughout this section, we will use a common set of general symbols:
- A generic acid will be represented as HA, where ‘H’ is the acidic proton and ‘A’ is the anion. A generic conjugate acid will be represented as HB+.
- A generic base will be represented as B. A generic conjugate base will be represented as A−.
Quick Reference
The definitions and types of Acids and Bases can be conveniently accessed in the “Quick Reference” section.
The Arrhenius Definition: The Role of Water
The earliest modern definition, proposed by Svante Arrhenius, focuses exclusively on the behavior of substances in aqueous solution.
An Arrhenius acid is a substance that increases the concentration of hydronium ions (H3O+) when dissolved in water. \[ {\color{green}\mathrm{HA}}\mathrm{(aq)} + \mathrm{H_2O(l)} \rightleftharpoons \mathrm{H_3O^+(aq)} + {\color{red}\mathrm{A^-}}\mathrm{(aq)} \]
An Arrhenius base is a substance that increases the concentration of hydroxide ions (OH−) when dissolved in water. This can happen by dissociation (for ionic hydroxides) or by reaction with water. \[ \begin{aligned} \text{(by dissociation)} \quad \mathrm{NaOH}\mathrm{(s)} &\xrightarrow{\mathrm{H_2O(l)}} \mathrm{Na^+(aq)} + {\color{red}\mathrm{OH^-}}\mathrm{(aq)} \\[1.5ex] \text{(by reaction)} \quad {\color{red}\mathrm{B}}\mathrm{(aq)} + \mathrm{H_2O(l)} &\rightleftharpoons \mathrm{OH^-(aq)} + {\color{green}\mathrm{HB^+}}\mathrm{(aq)} \end{aligned} \]
While useful, the Arrhenius theory is limited because it only applies to reactions in water.
The Brønsted-Lowry Definition: The Proton Transfer
The Brønsted-Lowry theory provides a more general and powerful definition based on the transfer of a proton (a hydrogen ion, H+).
- A Brønsted-Lowry (B-L) acid is a substance that can donate a proton.
- A Brønsted-Lowry (B-L) base is a substance that can accept a proton.
An acid-base reaction, in this view, is simply a proton transfer. This theory also introduces the crucial concept of conjugate acid-base pairs. When a B-L acid donates its proton, it becomes a conjugate base. When a B-L base accepts a proton, it becomes a conjugate acid.
\[ \begin{array}{ccccccc} {\color{green}\mathrm{HA}} & \!\!\!+\!\!\! & {\color{red}\mathrm{H_2O}} & \!\!\!\rightleftharpoons\!\!\! & {\color{green}\mathrm{H_3O^+}} & \!\!\!+\!\!\! & {\color{red}\mathrm{A^-}} \\ \small\text{B-L acid} & & \small\text{B-L base} & & \small\text{conjugate acid} & & \small\text{conjugate base} \end{array} \]
Notice that water can act as either an acid or a base, a property known as being amphoteric.
Because the Brønsted-Lowry theory is not limited to aqueous solutions, it can also describe reactions between gases, like the formation of ammonium chloride:
\[ \begin{array}{ccccc} {\color{green}\mathrm{HCl}}(\mathrm{g}) & \!\!\!+\!\!\! & {\color{red}\mathrm{NH_3}}(\mathrm{g}) & \!\!\!\rightleftharpoons\!\!\! & {\color{green}\mathrm{NH_4Cl}}(\mathrm{s}) \\ \small\text{B-L acid} & & \small\text{B-L base} & & \small\text{salt} \end{array} \]
The concept of conjugate pairs is a central idea in the Brønsted-Lowry theory. Every B-L acid-base reaction contains two such pairs.
A conjugate acid-base pair consists of two species that are related to each other by the gain or loss of a single proton (H+).
- When a Brønsted-Lowry acid donates a proton, the species that remains is its conjugate base. \[ \underset{\small\text{acid}}{\color{green}\mathrm{HA}} \!\!\quad\!\! \xrightarrow{\text{- H}^{+}} \!\!\quad\!\! \underset{\small\text{conjugate base}}{\color{red}\mathrm{A^-}} \]
- When a Brønsted-Lowry base accepts a proton, the new species that is formed is its conjugate acid. \[ \underset{\small\text{base}}{\color{red}\mathrm{B}} \!\!\quad\!\! \xrightarrow{\text{+ H}^{+}} \!\!\quad\!\! \underset{\small\text{conjugate acid}}{\color{green}\mathrm{HB^+}} \]
Let’s identify the two pairs in the reaction of a generic acid with water:
\[ {\color{green}\mathrm{HA}} + {\color{red}\mathrm{H_2O}} \rightleftharpoons {\color{green}\mathrm{H_3O^+}} + {\color{red}\mathrm{A^-}} \]
| Conjugate Pair | Acid | Base |
|---|---|---|
| Pair 1 | \({\color{green}\mathrm{HA}}\) | \({\color{red}\mathrm{A^-}}\) |
| Pair 2 | \({\color{green}\mathrm{H_3O^+}}\) | \({\color{red}\mathrm{H_2O}}\) |
In this reaction, water acts as the base, accepting a proton from HA to form the hydronium ion, H3O+
The Lewis Definition: The Electron Pair Transfer
The most general of the three theories, proposed by G. N. Lewis, defines acids and bases not by their behavior with protons, but by their interaction with electron pairs.
- A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond.
- A Lewis base is a substance that can donate a pair of electrons to form a covalent bond.
This definition is the most inclusive because it can explain the acidity and basicity of molecules that do not contain hydrogen at all. A classic example is the reaction between boron trifluoride and ammonia:
\[ \begin{array}{ccccc} {\color{green}\mathrm{BF_3}} & \!\!\!+\!\!\! & {\color{red}\mathrm{:NH_3}} & \!\!\!\rightarrow\!\!\! & \mathrm{F_3B{-}NH_3} \\ \small\text{Lewis acid} & & \small\text{Lewis base} & & \small\text{adduct} \end{array} \]
Here, the Lewis base (ammonia) donates its lone pair of electrons to the electron-deficient Lewis acid (boron trifluoride), forming a coordinate covalent bond in a product called an adduct.
Strong vs. Weak Acids and Bases
The terms “strong” and “weak” describe the extent to which an acid or base dissociates or reacts in solution.
- A strong acid or base is a strong electrolyte. It dissociates completely in solution, meaning the equilibrium lies very far to the right (product-favored).
- A weak acid or base is a weak electrolyte. It only partially dissociates, meaning the equilibrium lies to the left (reactant-favored), and a significant amount of the unreacted molecular form remains in solution.
You may notice that acids seem to fit in a gray area between molecular and ionic compounds. So, what are they? The answer depends entirely on whether you are describing the pure substance or the aqueous solution.
Pure Acids are Molecular Compounds: In their pure, undissolved state (e.g., a tank of hydrogen chloride gas, HCl(g), or a bottle of pure, “glacial” acetic acid, CH3COOH(l)), acids are unambiguously molecular compounds. Their atoms are held together by covalent bonds.
Aqueous Solutions Contain Ions: The defining characteristic of an acid is that it ionizes in water by reacting with it.
- Strong acids ionize completely, meaning the solution contains almost exclusively solvated ions (like H3O+ and Cl−) and behaves like a solution of an ionic compound.
- Weak acids only ionize slightly, meaning the solution contains mostly intact molecules in equilibrium with a small number of ions.
The Takeaway: For classification purposes, treat acids as their own unique category. They are molecular compounds by nature, but their chemical identity is defined by their ability to produce ions in water.
Common Acids and Bases
The following table lists the common strong and weak acids and bases that are essential to recognize in general chemistry.
Knowing the short list of common strong acids and strong bases by heart is one of the most important skills you can develop at this stage. It is a standard requirement in all university-level general chemistry courses.
Because strong acids and bases dissociate completely, we treat them differently when writing ionic equations. Being able to instantly recognize a substance as “strong” or “weak” is the key to correctly predicting the outcomes of aqueous reactions.
Use the Skill Drill: Acids and Bases tool to quiz yourself!
Biological Systems
Blood pH Regulation
- Concept: The bicarbonate buffer system maintains blood pH at ~7.4
- Connection: Same chemistry you learned about conjugate acid-base pairs
- Why It Matters: Without proper buffering, enzymes wouldn’t function and oxygen transport would fail
- Key Reaction: CO2(g) + H2O(l) ⇌ H2CO3(aq) ⇌ HCO3−(aq) + H3O+(aq)
Stomach Acid and Antacids
- Concept: Stomach contains HCl for digestion; antacids neutralize excess acid
- Connection: Classic acid-base neutralization you studied
- Everyday Application: Tums, Rolaids, and other antacids work on the same principles
- Reaction: CaCO3(s) + 2 HCl(aq) ⇌ CaCl2(aq) + H2O(l) + CO2(g)
Enzyme Function
- Concept: Most enzymes only work within narrow pH ranges
- Connection: Weak acid/base equilibria affect protein structure
- Why It Matters: Your body temperature regulation depends on enzyme pH optima
Environmental Chemistry
Acid Rain Formation
- Concept: SO2 and NOx gases react with water to form acidic solutions
- Connection: Forms Arrhenius acids in the atmosphere
- Impact: Damages buildings, harms aquatic life, affects forest health
- Reactions: SO3(g) + H2O(l) ⇌ H2SO4(aq) (strong acid)
Lake Neutralization
- Concept: Adding limestone (CaCO3) neutralizes acidic lakes
- Connection: Same neutralization chemistry as antacids, but on environmental scale
- Restoration: Brings pH back to levels where fish and plants can survive
Industrial Applications
Food Preservation
- Concept: Acidic environments prevent bacterial growth
- Connection: Vinegar (acetic acid) and citric acid create unfavorable pH for microbes
- Examples: Pickling vegetables, preserving fruits, salad dressings
- Household Chemistry: Why lemon juice prevents avocado browning
Chemical Manufacturing
- Concept: Many industrial processes require precise pH control
- Connection: Strong acids/bases used as catalysts and reagents
- Examples: Paper manufacturing, petroleum refining, pharmaceutical production
- Safety: Why acid-resistant materials and proper handling procedures are crucial
Cleaning Products
- Concept: Acids and bases break down different types of stains
- Connection: Vinegar (weak acid) removes mineral deposits; ammonia (weak base) removes grease
- Everyday Use: Descaling coffee makers, cleaning windows, removing soap scum
- Safety Note: Why you should never mix cleaning products!
Consumer Products
Carbonated Beverages
- Concept: CO2 dissolved in water forms carbonic acid (H2CO3)
- Connection: Forms weak acid that creates the “fizzy” sensation
- pH Effect: Most sodas have pH around 2.5–3.5 (quite acidic)
- Everyday Science: Why soda cans are lined and why mentos cause explosions
Batteries
- Concept: Many batteries rely on acid-base reactions
- Connection: Car batteries use sulfuric acid (strong acid) reactions
- Energy Production: Chemical energy ⇌ electrical energy
- Safety: Why battery acid is dangerous and requires protective equipment
Swimming Pool Maintenance
- Concept: Maintaining proper pH prevents algae growth and equipment corrosion
- Connection: Testing and adjusting pH using acids or bases
- Optimal Range: pH 7.2–7.8 for swimmer comfort and equipment protection
- Chemistry Balance: Balancing pH with chlorine effectiveness